How Do You Know if a Molecule Is Stable
ii.five: Rules for Resonance Forms
- Page ID
- 31387
After completing this department, you should be able to
- utilise the concept of resonance to explain structural features of molecules and ions.
- understand the relationship between resonance and relative stability of molecules and ions.
Rules for Drawing and Working with Resonance Contributors 
Recognizing, drawing, and evaluating the relative stability of resonance contributors is essential to understanding organic reaction mechanisms. When learning to draw and interpret resonance structures, there are a few basic guidelines to help. .
1) There is ONLY Ane Existent Structure for each molecule or ion. This real structure (the resonance hybrid) takes its grapheme from the average of all the private resonance contributors. When looking at a resonance contributors, we are seeing the verbal same molecule or ion depicted in unlike means. Resonance hybrids are really a single, unchanging structure.
Major resonance contributors of the formate ion
Representations of the formate resonance hybrid
ii) The resonance hybrid is more stable than any private resonance structures. Often, resonance structures correspond the movement of a charge between two or more atoms. The accuse is spread out amongst these atoms and therefore more stabilized. When looking at the picture in a higher place the resonance contributors represent the negative charge as existence on ane oxygen or the other. The resonance hybrid shows the negative accuse being shared equally between two oxygens. In the resonance hybrid, the negative charge is spread out over a larger part of the molecule and is therefore more stable.
3) Resonance contributors exercise non have to be equivalent. Because of this, resonance structures practice necessarily contribute equally to the resonance hybrid. The ii resonance structures shown below are not equivalent because one testify the negative charge on an oxygen while the other shows information technology on a carbon. Later, nosotros will evidence that the correspondent with the negative charge on the oxygen is the more than stable of the two. Also, this means that the resonance hybrid will not be an verbal mixture of the ii structures.
4) All resonance contributors must be right Lewis structures. Each atom should have a complete valence beat and be shown with correct formal charges. A carbocation (carbon with only six valence electrons) is the but immune exception to the valence beat rules. The structure below is an invalid resonance structure even though information technology only shows the movement of a pi bail. The resulting structure contains a carbon with ten electrons, which violates the octet dominion, making it invalid.
5) All resonance contributors must have the same molecular formula, the same number of electrons, and same internet charge. The molecules in the figure beneath are not resonance structures of the same molecule because then have unlike molecular formulas (C2H5NO Vs. C2H6NO). Also, the two structures have different net charges (neutral Vs. positive).
6) Resonance contributors but differ by the positions of pi bond and lone pair electrons. Sigma bonds are never broken or made, because of this atoms must maintain their same position. The molecules in the figure below are not resonance structures of the aforementioned molecule even though they have the same molecular formula (C3Hhalf-dozenO). These molecules are considered structural isomers because their difference involves the breaking of a sigma bond and moving a hydrogen atom.
Major and Minor Resonance Contributors
Equally previously state the true structure of a resonance hybrid is the combination of all the possible resonance structures. If the resonance structures are equal in stability they the contribute every bit to the structure of the hybrid. However, if the resonance structures have different stabilities they contribute to the hybrid's structure in proportions related to their relative stabilities. It tin exist said the the resonance hybrid'due south structure resembles the about stable resonance structure. Because of this it is of import to be able to compare the stabilities of resonance structures. In the example below, structure B is much less of import in terms of its contribution to the hybrid because it contains the violated octet of a carbocation. The relative stabilities of the two structures are and then vastly different that molecules which contain a C=O bond are nigh exclusively written in a grade like structure A. However, every bit will learn in chapter 19, the positively charged carbon created by structure B will explain how the C=O bond will react with electron rich species.
Rules for Estimating Stability of Resonance Structures
1. The resonance structures in which all atoms accept complete valence shells is more stable. This means most atoms have a full octet. In the example below structure A has a carbon atom with a positive charge and therefore an incomplete octet. Based on this criterion, structure A is less stable and is a more than pocket-size contributor to the resonance hybrid than structure B.
2. The structures with the to the lowest degree number of formal charges is more stable. Based on this, construction B is less stable considering is has two atoms with formal charges while structure A has none. Structure A would be the major resonance contributor.
3. The structures with a negative charge on the more electronegative atom will be more stable. The difference between the ii resonance structures is the placement of a negative charge. Structure B is the more than stable and the major resonance contributor, because information technology places the negative charge on the more electronegative oxygen.
4. The structures with a positive charges on the least electronegative atom (nearly electropositive) is more stable.
five. The structures with the to the lowest degree separation of formal charges is more stable. The merely difference between the two structures below are the relative positions of the positive and negative charges. In structure A the charges are closer together making information technology more stable.
6. Resonance forms that are equivalent take no difference in stability. When looking at the two structures below no deviation tin can be fabricated using the rules listed above. This means the ii structures are equivalent in stability and would make equal structural contributions to the resonance hybrid.
Examples of major and minor contributors
Case 1:
Case 2:
Example 3:
Carboxylate example
In the case of carboxylates, contributors A and B below are equivalent in terms of their relative contribution to the hybrid structure. Even so, there is also a third resonance correspondent C, in which the carbon bears a positive formal charge (a carbocation) and both oxygens are unmarried-bonded and bear negative charges.
Structure C makes a less of import contribution to the overall bonding picture of the group relative to A and B. How do we know that structure C is the 'pocket-sized' contributor? Apply the rules below
- The carbon in correspondent C does not have an octet. In full general, resonance contributors in which a carbon does non fulfill the octet rule are relatively less of import. (rule #ane)
- In structure C, there are merely iii bonds, compared to four in A and B. In general, a resonance structure with a lower number of total bonds is relatively less important. (dominion #2)
- Structure C also has more formal charges than are present in A or B. In general, resonance contributors in which there is more/greater separation of charge are relatively less important. (rule #iii)
- Structures A and B are equivalent and will be equal contributors to the resonance hybrid. (rule #5).
- The resonance contributor in which a negative formal charge is located on a more electronegative atom, usually oxygen or nitrogen, is more than stable than one in which the negative charge is located on a less electronegative cantlet such as carbon. An instance is in the upper left expression in the side by side effigy. (rule #4)
Draw the major resonance contributor of the structure beneath. Include in your figure the appropriate curved arrows showing how yous got from the given structure to your structure. Explain why your correspondent is the major one. In what kind of orbitals are the two solitary pairs on the oxygen?
Solution
In the construction in a higher place, the carbon with the positive formal charge does not have a consummate octet of valence electrons. Using the curved arrow convention, a alone pair on the oxygen tin exist moved to the adjacent bond to the left, and the electrons in the double bail shifted over to the left (see the rules for drawing resonance contributors to convince yourself that these are 'legal' moves).
The resulting resonance contributor, in which the oxygen bears the formal charge, is the major one considering all atoms accept a complete octet, and at that place is one boosted bond drawn (resonance rules #ane and #2 both apply). This system can be thought of as iv parallel 2p orbitals (one each on C2, C3, and C4, plus one on oxygen) sharing 4 pi electrons. 1 lone pair on the oxygen is in an unhybridized 2p orbital and is role of the conjugated pi system, and the other is located in an spii orbital.
Also note that 1 additional contributor tin exist fatigued, only it is also small because it has a carbon with an incomplete octet:
Exercises
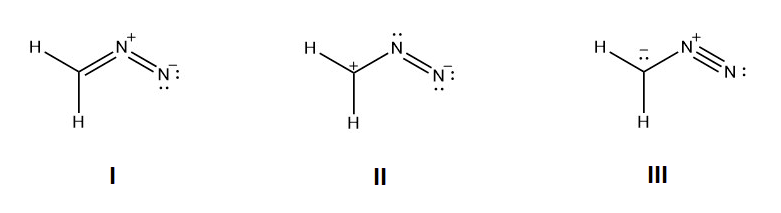
ane) For the following resonance structures delight rank them in social club of stability. Indicate which would be the major contributor to the resonance hybrid.
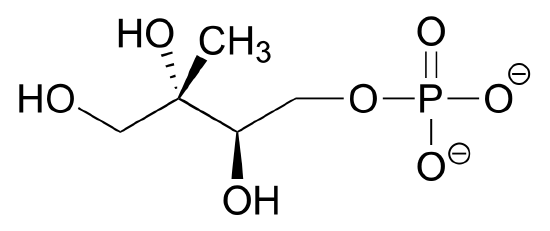
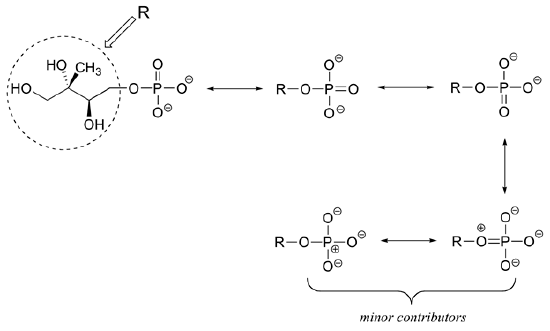
2) Draw four boosted resonance contributors for the molecule beneath. Label each ane every bit major or minor (the structure beneath is of a major contributor).
3) Draw three resonance contributors of methyl acetate (an ester with the structure CH3COOCHiii), and club them according to their relative importance to the bonding motion picture of the molecule. Explain your reasoning.
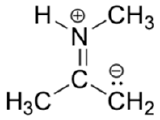
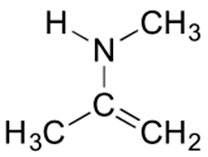
4) Beneath is a minor resonance contributor of a species known as an 'enamine', which we will study more in Department 19.eight (formation of enamines) Department 23.12 (reactions of enamines). Draw the major resonance contributor for the enamine, and explicate why your contributor is the major ane.
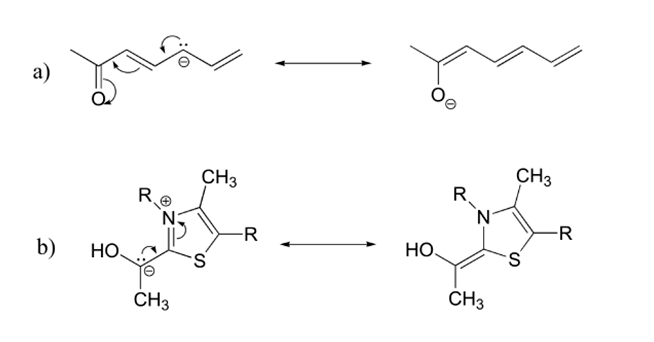
5) Draw the major resonance contributor for each of the anions beneath:
Solutions
1) Structure I would be the about stable because all the not-hydrogen atoms accept a total octet and the negative accuse is on the more electronegative nitrogen. Construction Iii would exist the next in stability because all of the non-hydrogen atoms accept full octets. Structrure 2 would be the least stable because it has the violated octet of a carbocation.
two)
3)
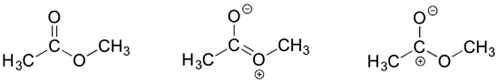
The contributor on the left is the most stable: there are no formal charges.
The contributor on the correct is least stable: there are formal charges, and a carbon has an incomplete octet.
The correspondent in the centre is intermediate stability: there are formal charges, but all atoms accept a complete octet.
four) This contributor is major because there are no formal charges.
v)
Exercises
Recognizing Resonance
Resonance contributors involve the 'imaginary movement' of pi-bonded electrons or of lone-pair electrons that are next to (i.e. conjugated to) pi bonds. You can never shift the location of electrons in sigma bonds – if you show a sigma bail forming or breaking, you are showing a chemical reaction taking place. Likewise, the positions of atoms in the molecule cannot change between 2 resonance contributors.
Considering benzene will announced throughout this form, it is of import to recognize the stability gained through the resonance delocalization of the half-dozen pi electrons throughout the six carbon atoms. Benzene as well illustrates one way to recognize resonance - when it is possible to draw two or more equivalent Lewis structures. If we were to draw the structure of an aromatic molecule such equally 1,ii-dimethylbenzene, there are two ways that we could draw the double bonds:
Which fashion is correct? There are two simple answers to this question: 'both' and 'neither ane'. Both ways of drawing the molecule are equally adequate approximations of the bonding picture for the molecule, only neither one, by itself, is an accurate motion-picture show of the delocalized pi bonds. The two alternative drawings, even so, when considered together, give a much more accurate picture than either 1 on its ain. This is because they imply, together, that the carbon-carbon bonds are not double bonds, not single bonds, simply about halfway in between.
When information technology is possible to draw more than ane valid structure for a compound or ion, nosotros have identified resonance contributors: two or more than different Lewis structures depicting the aforementioned molecule or ion that, when considered together, do a ameliorate job of approximating delocalized pi-bonding than any single structure. Past convention, resonance contributors are linked by a double-headed arrow, and are sometimes enclosed by brackets:
In order to brand information technology easier to visualize the difference between two resonance contributors, small, curved arrows are often used. Each of these arrows depicts the 'motility' of 2 pi electrons. In the drawing of resonance contributors, nonetheless, this electron 'movement' occurs only in our minds, as nosotros try to visualize delocalized pi bonds. Nevertheless, use of the curved arrow annotation is an essential skill that yous will need to develop in drawing resonance contributors.
The depiction of benzene using the ii resonance contributors A and B in the figure in a higher place does not imply that the molecule at one moment looks like structure A, then at the adjacent moment shifts to expect like structure B. Rather, at all moments, the molecule is a combination, or resonance hybrid of both A and B.
Caution! It is very important to exist clear that in drawing two (or more) resonance contributors, nosotros are not drawing two different molecules: they are simply different depictions of the verbal same molecule. Furthermore, the double-headed resonance arrow does Not hateful that a chemical reaction has taken identify.
Benzene is often fatigued as just ane of the two possible resonance contributors (it is assumed that the reader understands that resonance hybridization is implied). However, sometimes benzene will be drawn with a circle inside the hexagon, either solid or dashed, as a way of drawing a resonance hybrid.
Examples of Resonance
Molecules with a Unmarried Resonance Configuration
Example one:
Example two:
Example 3:
Instance 4:
The above resonance structures show that the electrons are delocalized within the molecule and through this process the molecule gains actress stability. Ozone with both of its opposite formal charges creates a neutral molecule and through resonance it is a stable molecule. The actress electron that created the negative charge one terminal oxygen can be delocalized by resonance through the other terminal oxygen.
Benzene is an extremely stable molecule due to its geometry and molecular orbital interactions, only near importantly, due to its resonance structures. The delocalized electrons in the benzene ring make the molecule very stable and with its characteristics of a nucleophile, information technology volition react with a stiff electrophile only and afterwards the first reactivity, the substituted benzene will depend on its resonance to direct the next position for the reaction to add a 2nd substituent.
Molecules and ions with more than one resonance class:
Some structural resonance conformations are the major correspondent or the dominant forms that the molecule exists. For example, if nosotros look at the in a higher place rules for estimating the stability of a molecule, we see that for the third molecule the first and 2nd forms are the major contributors for the overall stability of the molecule. The nitrogen is more electronegative than carbon so, it tin handle the negative charge more carbon. A carbon with a negative accuse is the to the lowest degree favorable conformation for the molecule to exist, then the last resonance form contributes very little for the stability of the Ion.
Hybrid Resonance
The different resonance forms of the molecule help predict the reactivity of the molecule at specific sites.
The Hybrid Resonance forms evidence the different Lewis structures with the electron been delocalized. This is very of import for the reactivity of chloro-benzene considering in the presence of an electrophile it will react and the germination of another bond will be directed and decide by resonance. The lone pair of electrons delocalized in the aromatic substituted ring is where information technology can potentially form a new bail with an electrophile, every bit information technology is shown in that location are three possible places that reactivity tin take identify, the first to react will take place at the para position with respect to the chloro- substituent then to either ortho- position.
macandiehaddince1971.blogspot.com
Source: https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Map%3A_Organic_Chemistry_(McMurry)/02%3A_Polar_Covalent_Bonds_Acids_and_Bases/2.05%3A_Rules_for_Resonance_Forms


0 Response to "How Do You Know if a Molecule Is Stable"
Post a Comment